-
Chaperon Proteins and Disulfide Bond Isomerase, Chaperon Proteins and Disulfide Bond Isomerase, Enzymes, Protein Purification
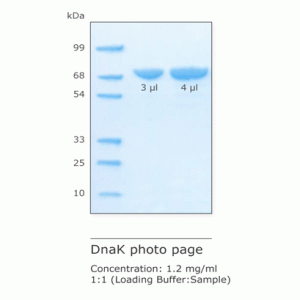
DnaK (HSP70)
DnaK is the prokaryotic analogue of eukaryotic Hsp70, a member of the heat shock proteins. It assists the protein assembly, folding, and translocation.
-
Chaperon Proteins and Disulfide Bond Isomerase, Chaperon Proteins and Disulfide Bond Isomerase, Enzymes, Protein Purification
GroEL
The bacterial chaperonin GroEL is a double toroidal assembly, which together with the action of the ring-shaped oligomeric cochaperonin, GroES, facilitates protein folding in an ATP dependent manner.
-
Chaperon Proteins and Disulfide Bond Isomerase, Chaperon Proteins and Disulfide Bond Isomerase, Enzymes, Protein Purification
GroES
GroES protein is a chaperonin family protein that works in conjunction with GroEL to facilitate proper protein folding. GroES consists of 7 subunits with a molecular weight of 10.4kDA. The…
-
Chaperon Proteins and Disulfide Bond Isomerase, Chaperon Proteins and Disulfide Bond Isomerase, Enzymes, Protein Purification
PDI1 (yeast)
Recombinant yeast PDI is produced in E.coli is as single, non-glycosylated, polypeptide chain containing 503 amino acids and having a molecular mass of 62.4 kDa. The PDI has a…